Abstract
Background: Although the cohort and registry data support low-dose (LD; 50 IU/kg once every other day) and intermediate-dose (MD; 100 IU/kg daily) immune tolerance induction (ITI) are almost equally effective in inducing tolerance in people with severe hemophilia A, with inhibitors (PwHI)1-3, there has been no prospective randomized trials, especially in China. Here, we present the protocol and results of the primary analysis in LOME (ChiCTR2200056603).
Methods LOME is a multicenter, prospective, randomized comparison of LD and MD ITI, in severe hemophilia A (HA) patients (FVIII level < 1%) with peak historical inhibitor ≥ 5 and < 200 Bethesda Unite (BU/ml). In the first year of recruitment (Part A), patients with age ≤ 8 years at time of randomized will be included. In the second year of recruitment (Part B), patients with age ≤ 12 years at time of randomized will be included. Patients have received ITI therapy before randomization will be excluded.
Subjects were randomized 1:1 to LD or MD ITI with the first domestic recombinant factor VIII (rFVIII) (Omfiloctocog alfa, SCT800) approved in China. Factor III (FVIII) inhibitor screening, ITI monitoring and dose tapering will be based on the consensus from United Kingdom Hemophilia Center Doctors' Organization (UKHCDO)4.
If (a) there is an upward trend in titer over 3-6 months period; or (b) fall in chromogenic Bethesda titer of less than 20% in 6 months period after initial peak inhibitor titer; or (c) titer ≥ 200BU/ml during ITI therapy; or (d) spontaneous bleeding > 1 monthly over 3-6 months period, modify the regimen as below:
In the LD arm, increase to 100IU/kg daily;
In the MD arm, rituximab 375 mg/m2 weekly × 4 weeks (maximum 600 mg) and prednisone 2 mg/kg daily × 1 month (maximum 60 mg, tapering over 3 months) would be added.
Once the patient had achieved Success, taper dose as below:
In the LD arm, the FVIII dose would be reduced slowly to 25IU/kg three times a week for continuing prophylaxis;
In the MD arm, the FVIII dose would be reduced slowly to LD ITI, then same as LD arm.
The primary endpoints are ITI outcomes (Success: negative inhibitor titer and FVIII recovery ≥ 66% of expected) and the time taken to ITI Success, in PwHI with LD ITI compared to those with MD ITI.
Key secondary endpoints include annualized bleeding rate (ABR), annualized joint bleeding rate (AJBR) for any bleeds and treated bleeds; joint and bone health assessed by Hemophilia Joint Health Score 2.0 (HJHS 2.0) and ultrasound; quality of life measured by the Canadian Hemophilia Outcomes-Kids Life Assessment Tool 2.0 (CHO-KLAT 2.0) and numbers of days out of school; average consumption cost (per kg body weight) was calculated based on the intermediate number of treatment doses consumed to achieve Success. This included the cost of rFVIII and rituximab for ITI, prothrombin complex concentrate (PCC) or recombinant activated factor VII (rFVIIa) for treatment of breakthrough bleed, and intravenous immunoglobulin (IVIG) for infection prevention in patients receiving immunosuppressants. Safety was also a secondary endpoint.
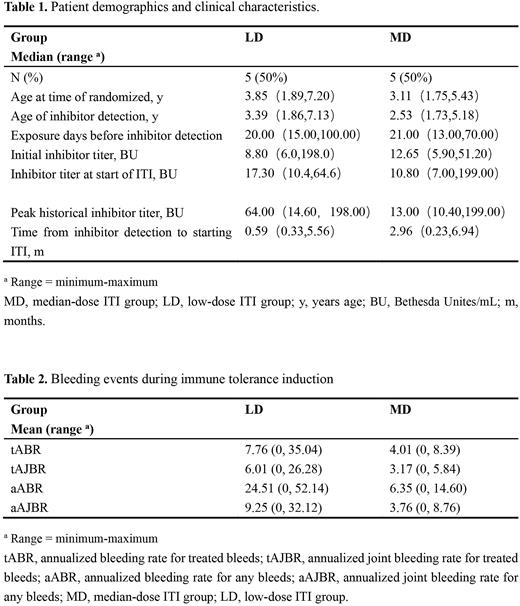
Results: Ten subjects were randomized into the study with median follow-up time of 1.64 [range (minimum, maximum), 0.23, 3.91] months in LD arm, and 2.66 (0.26,3.91) months in MD arm. Baseline includes age at time of randomized, age of inhibitor detection, exposure days before inhibitor detection, initial inhibitor titer, inhibitor titer at start of ITI and so on, between two arms are summarized in Table 1.
Twenty percent (1/5) in MD arm achieved Success after 7 weeks of treatment and still maintained until the latest follow-up. No one achieved Success in LD arm.
The bleeds between the treatment arms are summarized in Table 2. Nearly 2-fold difference in mean ABR and AJBR for treated bleeds, between treatment arms, and 3- to 4-fold in ABR and AJBR for any bleeds.
Conclusions: It is planned to enroll 40 patients in Part A and 60 patients in Part B, who will be followed for up to 2 years. The study is ongoing and, currently, 10/40 (25%, in Part A) PwHI from 4 HTCs have been included. LOME study is the first, multicenter, prospective, randomized trial of LD vs MD ITI with domestic rFVIII (Omfiloctocog alfa, SCT800) in Chinese PwHI. This study is likely to contribute with the choice of optimal ITI regimen.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.